Published On Feb 10, 2023
Claim your SPECIAL OFFER for MagellanTV here: https://try.magellantv.com/arvinash Start your free trial TODAY so you can watch “North Korea vs USA" about how North Korea has gotten international help in creating a Nuclear bomb: https://www.magellantv.com/video/nort...
RECOMMENDED VIDEOS:
Quantum tunneling: • Is Quantum Tunneling the Key to Life ...
How a nuclear bomb works: • Nuclear Bomb: How it Works in detail....
How Fusion in the Sun works: • Why Does the SUN SHINE? The Quantum M...
WANT MORE ANIMATIONS? Join our PATREON. Your generosity helps us create them:
/ arvinash
CHAPTERS:
0:00 Become dangerously interesting
1:29 Atomic components & Forces
3:55 What is an isotopes
4:10 What is Nuclear Decay
5:45 What is Radioactivity - Alpha Decay
6:31 Natural radioactivity - Beta & Gamma decay
9:03 What is half-life?
9:41 Nuclear fission
10:48 Nuclear fusion
SUMMARY
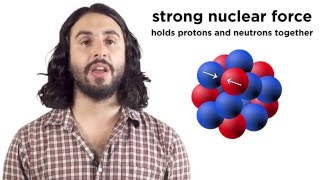
In this video, I summarize All Nuclear Physics in about 10 minutes. Atoms are made of a positively charged nucleus surrounded by negatively charged electrons. The nuclei of atoms are made up of protons and neutrons. These are called nucleons. Because all protons have the same charge, they repel. And force can be calculated using coulombs law. Two protons separated by the width of a neutron repel with a force of 60 Newtons, or 12 lbs!
But what keeps them from flying apart is an even stronger force the strong nuclear force. It's about 100X stronger than electromagnetism. But this force only operates at very small distances, about the width of a proton. And it only operates within and between nucleons. Electrons, photons, and neutrinos are not affected by it. It's like Velcro. Nucleon stick together when really close, but have no affect when far apart.
The number of protons in an element is solely responsible for its chemical and physical properties. A given element or atom can have the same number of protons, but different numbers of neutrons. These are called isotopes of the element. They have exactly the same chemical properties and differ only in mass.
Stable nuclei consist of roughly the same number of protons and neutrons. The neutrons serve to provide additional strong force needed to keep the nucleus stable. Without neutrons, not even two protons can be held together against their repulsion.
Free protons are stable, but free neutrons are not stable. Lone neutrons decay into a proton, an electron, and an antineutrino within 15 minutes. But inside a nucleus they remain stable because it is energetically unfavorable for them to decay.
If a nucleus is very large, or has an excess number of protons or neutrons, this causes alpha decay, which is a helium nucleus consisting of 2 protons and two neutrons. This is what we call radioactivity.
There are three forms of natural radioactivity, alpha, beta and gamma-decay. A beta-particle is a high-energy electron. This occurs in very large nuclei when a neutron decays even though it is in the presence of protons. This gives off an electron and an antineutrino. This electron is the beta-particle.
A gamma-particle is a high-energy photon. Gamma-rays are usually emitted by excited nuclei that have been created after either an alpha or beta decay. These nuclear processes release high energy photons is because they involve the strong force with is very energetic.
Alpha-particles can be stopped by a thin piece of paper. Beta-particles can penetrate your skin, but can be stopped by a sheet of aluminum foil. But gamma-rays can penetrate through an inch of lead.
A radioactive nucleus is characterized by its “half-life.” What this means is that if I have a 16 atoms, with a half-life of 1 week, then one week later I will have ½ or 8 atoms remaining. In 2 weeks, I will have 4 and so on. The half-life is a statistical concept, and we can't predict in advance which specific atoms will decay.
If a large nucleus, like some isotopes of uranium is hit by a particle, usually a neutron, then it will split into two smaller nuclei. This is called nuclear fission. If the total mass of the two smaller nuclei is less than that of the uranium before it was hit, the missing mass is turned into energy via E = mc2. This is called fission.
If there is enough fissionable nuclei in high enough concentration, then it is possible for the thrown off neutrons to, in turn, fission more nuclei, creating a chain reaction. This is the mechanism behind an atomic bomb.
#nuclearphysics
Fusion happens when two small nuclei such as hydrogen, which consists of only a single proton, can be brought close enough together that they fuse into a single nucleus. Fusion is very difficult to achieve, because the protons strongly repel each other. Only gases heated to millions of degrees Celsius have atoms moving fast enough to fuse. In the sun this process is easier because of the assistance of gravitational pressure in the core. Quantum tunneling also plays a role.