Published On Premiered Aug 29, 2020
Go to https://brilliant.org/ArvinAsh you can sign up for free. And also, the first 200 people will get 20% off their annual premium membership.
Video on QM of Atoms which may clarify some concepts: • The Quantum Mechanical model of an at...
Why do atoms combine to form molecules? The quantum mechanics of chemical bonds and chemistry. How is it that we can have so many chemical substances with only about 100 relatively simple building blocks, called atoms? This is because atoms are rarely found alone. They are mostly found in combinations with other atoms, through the process of chemical bonding.
Why does this happen? Why is the universe not full of just atoms floating around? The answer to this important question lies in understanding the role that energy plays in the formation of molecules, and its roots in quantum mechanics. All natural systems tend to adopt a state of lowest energy.
A marble at the top of a hill has high potential energy due to gravity. If given the opportunity, it will roll naturally to the bottom of the hill where it will have a lower potential energy.
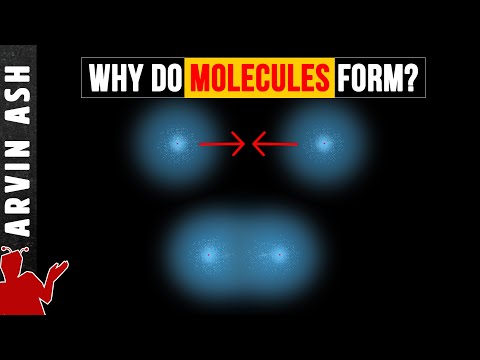
The hydrogen atom by itself will be in its lowest energy state, called the ground state. But when a second hydrogen atom is introduced, some things begin to happen. Both atoms are in their respective ground state. But as they come closer to each other, first, the electrons since they are both negatively positively charged repel each other, but the electron of hydrogen atom 1 also starts to get affected by the positive charge of the proton in hydrogen atom 2. Similarly the electron of atom 2 starts to get attracted to the proton of atom 1.
So the electrons of each of the 2 atoms tend to get pulled slightly to the other one’s proton. And if they get close enough, the cloud begins to spread to the space between the two atoms. if the atoms get too close, then the protons begin to repel each other and push each other apart. So there is an optimal distance that the two protons prefer to be in.
Shouldn’t the electron clouds be repelling each other, and not allow them to get anywhere near each other? No, there are other interactions that affect the energy of the system:
To calculate the lowest energy of this two atom system, or molecule of hydrogen, we have to take into account:
1) The kinetic energy each atom
2) The potential energy between the two protons
3) The potential energy between the two electrons
4) The potential energy between each electron and each proton
The sum of the possible outcomes of kinetic and potential energy of this entire system in quantum mechanics is referred to the Hamiltonian, represented by capital H. This Hamiltonian is an operator corresponding to the energy of the system, and once you plug it into the time-independent Schrodinger equation, you can solve to get possible values for energy. This is not a trivial equation to solve. But it can be represented for simplicity by the morse potential graph.
The energy two atoms system is less than the energy of two separate one atom systems. This is the reason if a bunch of hydrogen atoms are near each other, they will naturally combine to form a molecule of H2 rather than float around by themselves. This sharing of electrons by two atoms of hydrogen is called a covalent bond.
Not all atoms form bonds with atoms of their own kind, nor with just any other atom. All the substances strive to achieve remarkable stability by sharing or having magical numbers of electrons – 2, 10, 18, 36, 54, or 86 electrons in so called shells around the nucleus of atoms. These numbers correspond to the number of electrons contained in the 6 naturally occurring noble gases. These are inert elements because they already contain the number of electrons needed to form highly stable shells around the nucleus.
Other elements strive to contain a full set of electron in their outer shell called the valence shell. Any element with an unfilled outer shell has a much higher chemical potential energy than these noble gases.
But what's so special about these numbers? Chemists will say, all atoms strive to form a valence set of electrons. And this attractive force for atoms to share electrons in order to form a full valence shell, is balanced by the repulsive forces of their electron clouds and protons.
#hamiltonian
#schrodingerequation
Quantum mechanically, it all has to do with potential energy of multi atom systems. The Schrodinger equation and the Pauli exclusion principle are the underlying principles.