Published On Dec 17, 2016
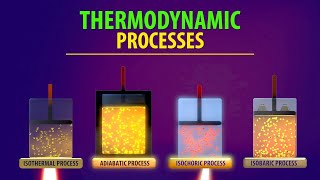
This physics video tutorial explains the concept of the first law of thermodynamics. It shows you how to solve problems associated with PV diagrams, internal energy, heat, and work. It addition, it provides plenty of examples and practice problems associated with isothermal, isochoric or isovolumetric, isobaric, and adiabatic processes.
Open Vs Closed Vs Isolated System: • Open System, Closed System and Isolat...
First Law of Thermodynamics:
• First Law of Thermodynamics, Basic In...
Isobaric Process:
• Isobaric Process Thermodynamics - Wor...
Isochoric Process:
• Isochoric Process Thermodynamics - Wo...
Isothermal Process:
• Isothermal process Thermodynamics - ...
Internal Energy of an Ideal Gas:
• Internal Energy of an Ideal Gas - Mol...
_________________________
Adiabatic Process:
• Adiabatic Process - Work, Heat & Inte...
PV Diagrams:
• PV Diagrams, How To Calculate The Wor...
2nd Law of Thermodynamics:
• Second Law of Thermodynamics - Heat E...
Heat Engines:
• Heat Engines, Thermal Efficiency, & E...
Converting Heat Into Electricity:
• Thermodynamics - Converting Heat Ener...
________________________
Carnot Cycle:
• Carnot Cycle & Heat Engines, Maximum ...
Otto Cycle:
• Otto Cycle of Internal Combustion Eng...
Refrigerators and Heat Pumps:
• Refrigerators, Heat Pumps, and Coeffi...
Entropy:
• Entropy Change For Melting Ice, Heati...
Heat Engines and Refrigerators Review:
• Carnot Heat Engines, Efficiency, Refr...
Physics 1 Review:
https://www.video-tutor.net/physics-b...
Chemistry Review:
https://www.video-tutor.net/chemistry...
Here is a list of topics:
1. First Law of Thermodynamics - Energy Transfer
2. Internal Energy, Heat, and Mechanical Work
3. System vs Surroundings
4. Sign Conventions for Q, Heat Absorbed vs Heat Energy Released
5. Work done on the system vs work done by the system
6. Positive Work - Gas Expansion vs Negative Work - Compression
7. Open System, Closed System and Isolated System
8. Isobaric Process - Constant Pressure
9. Work Done at Constant Pressure Formula / Equation
10. Units of Pressure - Converting atm to Pa
11. Units of Volume - m^3 and L conversion
12. Converting L and atm into Joules
13. PV Diagrams - Work Done = Area Under the Curve or Area of the Shaded Region
14. Cyclic Process, Q=W, Work is Positive for Clockwise Rotation and Negative for Counter Clockwise Direction
15. Ideal Gas Law, PV=nRT
16. Charles Law - Temperature and Volume Relationship
17. Converting Celsius to Kelvin Temperature
18. Heat Transferred During Isobaric Process Formula
19. Isochoric Process - Work Done is Zero
20. Isovolumetric Process - Constant Volume, Internal Energy, and Heat Transferred Equation
21. Thermodynamics Formula Sheet Summary
22. Molar Heat Capacity at Constant Pressure and Constant Volume
23. Monatomic Gases - He, Ne, and Ar - Helium, Neon, and Argon
24. Diatomic Gases - N2, O2, and H2
25. Cv and Cp values for ideal monatomic, diatomic and polyatomic gases
26. Gamma Ratio, Cp/Cv formula
27. Isochoric Process - Pressure and Temperature Formula
28. Combined Gas Law Equation
29. Isothermal Process - Constant Temperature
30. Work Done During an Isothermal Process - Natural Logarithms Derivation - Area under Curve
31. Boyle's Law - Pressure and Volume Relationship
32. Adiabatic Process - No Heat Transferred - Q=0
33. Adiabatic Expansion - Cooling Effect of an Ideal Gas
34. Adiabatic Compression - Increase in Temperature - No spark plugs needed in an internal combustion engine of this type
35. Work, Pressure, Volume and integration Formula, Calculus - Isothermal Process
36. Pressure and Volume Formula With Gamma Ratio
37. Temperature and Volume Equation With Gamma Ratio - Adiabatic Process
38. Internal Energy - State Function - Independent of Path
39. Work and Heat - Not a State Function, Path Dependent
40. PV diagram problems, work, heat, and internal energy calculations