Published On Mar 30, 2024
In this video, we look at decomposition reactions, a fundamental concept in general chemistry, and focus on predicting the products of these reactions.
⭐ Full courses at: / @wbreslyn
Useful Tools:
🔹Finding the Charge on Ions: • Figuring out the Charge on Ions
🔹Predicting Decomposition Rxns: https://youtu.be/ MIg8LGlYBbQ
🔹Predicting Combination Rxns: • Predicting Products of Combination Re...
🔹Types of Chem Reactions Playlist: • Types of Reactions and Predicting Pro...
🔹Balancing Equations: • How to Balance Chemical Equations in ...
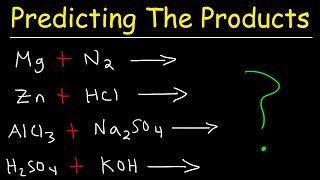
Basic Binary Decomposition Reactions
We start with the simplest form of decomposition reactions. An example we cover is sodium chloride (NaCl) decomposing into sodium (Na) and chlorine gas (Cl2).
Decomposition of Metal Carbonates
Next, we explore how metal carbonates break down. We'll look at calcium carbonate (CaCO3), which decomposes into calcium oxide (CaO) and carbon dioxide (CO2).
Decomposition of Metal Hydroxides
Following that, our focus shifts to metal hydroxides. For instance, sodium hydroxide (NaOH) decomposes into sodium oxide (NaO) and water (H2O).
Decomposition of Oxides
Lastly, we examine the decomposition of oxides, such as sodium oxide (Na2O) breaking down into sodium (Na) and oxygen gas (O2).
By categorizing these decomposition reactions, we can identify patterns that help in predicting the products of these reactions. This video is designed to simplify these concepts for students at the general chemistry level.
Join this channel to get full access to Dr. B's chemistry guides:
/ @wbreslyn