Published On Mar 4, 2019
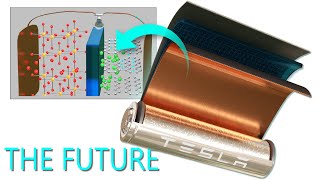
How many lithium-ion [Li-ion] batteries have you used today? Lithium-ion batteries are practically everywhere, but how do they work? Why are they rechargeable? And why do they die earlier and earlier in the day the more you use them? This episode will go deep into detail and explore the lithium-ion battery in your smartphone and answer each of these questions.
Do you want to support in-depth engineering and technology education? Support us on: / brancheducation
Website: https://www.branch.education
On Facebook: / brancheducation
On Twitter: / teddytablante
On Insta: / brancheducation
Or Join us on YouTube Memberships: / @brancheducation
Question: "I learned that the Anode is + and the Cathode is -. Why do you say the reverse?" Answer: Well, in electronic devices, by definition: the anode is where electrons leave the component. In a diode or LED, the anode, the anode is the + terminal, the cathode is - and current goes from + to -, and electrons go from - to +. However now, think of the battery that is powering that LED. When operating, electrons come out of the negative side, and by the definition that makes this the anode. Thus, for batteries the anode is - and the cathode is +. But, chemistry uses a different definition of 'the cathode is where species are reduced, and the anode is where species are oxidized'. And for a battery function vs recharging the anode and cathodes switch sides, but + and - designations stay the same.
TLDR: Anode and cathode are defined by more than just Anode is + and cathode is -.
Erratum:
9:29 Separator misspelled as Seperator
Website: www.branch.education
Twitter: @teddytablante
Made by Teddy Tablante
Table of Contents:
00:43 Section 1: How do Li-ion batteries work?
04:53 Section 2: How do Li-ion batteries recharge?
05:36 Section 2B: Additional details about Li-ion batteries.
07:18 Section 3: Why does your battery's max capacity reduce over time?
Background Understanding: Electrons
Key Branches from this video are: Electric Vehicle Batteries, Galvanic and Voltaic Cells, Chemical bonds & Electronegativity, and Lemon Batteries.
Animation built using Blender 2.79b https://www.blender.org/
Post with Adobe Premiere Pro and Adobe After Effects
Sound editing with Reaper
Work Cited:
J.B. Goodenough and Y. Kim, Chem. Mater., 22, 587 (2010).
Julien, Christian. And Alain Mauger and Ashok Vijh and Karim Zaghib, Lithium Batteries Science and Technology Springer, 2016.
Kazda, Tomas. Vanysek, Petr. "Lithium Batteries as Electrochemical Sources of Energy". The Electro Chemical Society. Fall 2016
Pinson, Matthew B. Bazant, Martin Z. "Theory of FEI Formation in Rechargeable Batteries, Capacity Fade, Accelerated Aging and Lifetime Prediction" MIT.
Romano, Linda Ph.D., "Improving Performance and Safety of Lithium-Ion Batteries: Characterizing Materials and Interfaces." EAG Laboratories 2017.
Strand, Dee. And Mark Jones (2016) "Chemistry of Hello: Lithium Ion Batteries" ACS Webinars Presentation 2016 Material Science Series
Wikipedia contributors. "Li-Ion Batteries." "Electrolyte." "Intercalation." "Electrical Energy." "Battery Electric Vehicle." "Battery Charger." "Rechargeable Battery." "Research in Lithium-ion Batteries." "Lithium-Silicon Battery." "Lithium Cobalt Oxide" Wikipedia, The Free Encyclopedia. Wikipedia, The Free Encyclopedia, March 3rd, 2019.
Blend Swap Model used: https://www.blendswap.com/
Blender Battery by Jafrem
Music Attribution in Order:
Morning Dew from YouTube Audio Library
Plaidness by Francis Preve
• Plaidness - Francis Preve | Royalty F...
Water Lillies from YouTube Audio Library
Marxist Arrow by Twin Musicom is licensed under a Creative Commons Attribution License https://www.youtube.com/watch?v=rBlLC...
Under Cover by Wayne Jones from YouTube Audio Library
Timelapsed Tides from YouTube Audio Library
Sunburst, Tobu & Itro is licensed under Creative Commons Attribution License
http://www.7obu.com
• Video
#How #LithiumIon #Batteries